
We have blocked rooms for the conference delegates at the conference venue:Īddress: Winthontlaan 4-6, 3526 KV Utrechtġ60euro include VAT and a full breakfast buffet excluding 6% city tax. Nederlandse Vereniging voor Kindergeneeskunde (NVK).Nederlandse Vereniging van Artsen voor Longziekten en Tuberculose (NVALT).The DLC is accredited by the following organizations for 6 points/day. For more information or sign up for your NRS-ERS membership, CLICK HERE.
#NVALT 2 REGISTRATION#
Sign up now to become an NRS member and save on your registration fee! The annual fee for NRS-ERS membership: € 80,. Non-specialist: Scientists/ Basisarts/ AIOS/ ANIOS/ LongFonds or patient representativeĪll prices are stated including 21% VAT. IMPORTANT NOTICE: The standard fees apply until 5 June. Registering after this date up to 22 June will be considered late-registration and incur an additional fee of €50 in every category.
The following standard fees apply according to your membership & function: We’re splitting off the 10.6 users so we can develop for Lion/Mountain Lion while. Depending on your OS version, you’ll then be pointed to an appropriate version for 10.6 or 10.7+.
#NVALT 2 DOWNLOAD#
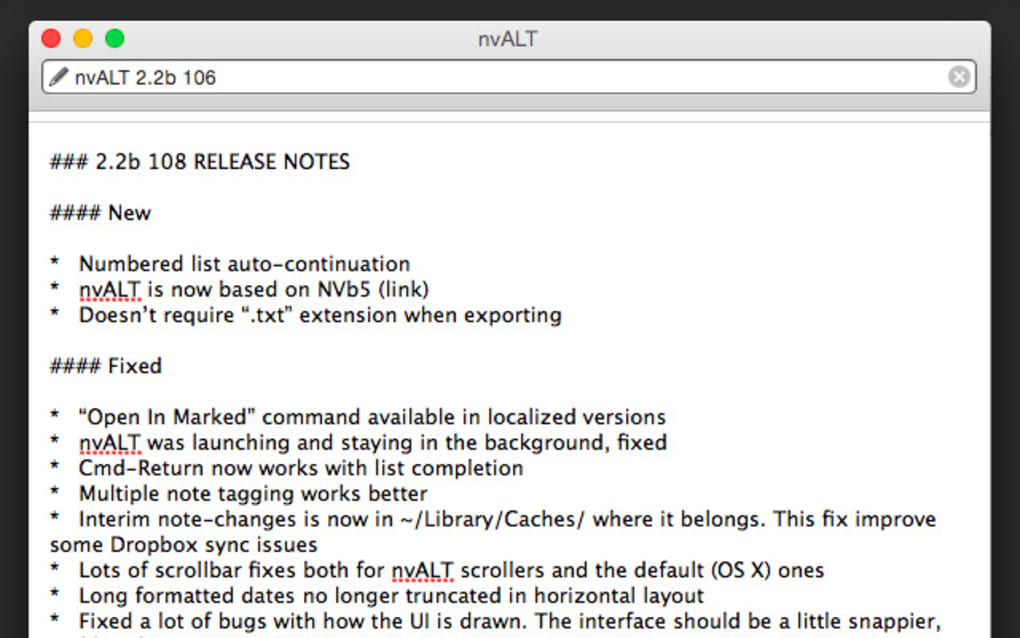
The first version you’ll get in your updater or direct download will be 2.2b106. All rights reserved.The DLC 2023 will take place 29-30 June at the Van der Valk Hotel Utrecht Programme The next (and hopefully last before official release) nvALT 2.2 beta is up. Published by Oxford University Press on behalf of the European Society for Medical Oncology. NSCLC carboplatin net benefit pemetrexed prediction. Individualized prediction of treatment effect could be used to guide shared decision-making by discriminating patients who benefit most, to improve clinical outcome. There is important heterogeneity in the effects on PFS of pemetrexed-carboplatin versus pemetrexed in pretreated patients with advanced non-squamous NSCLC. The effects of pemetrexed-carboplatin can be predicted for individual patients based on routinely available patient and tumor characteristics. Decision curve analysis confirmed that the model adequately identified patients who benefit from pemetrexed-carboplatin, as prediction-based treatment led to improvement in net benefit with regard to PFS and overall survival when assuming a treatment threshold of 0-5 months gain in PFS, compared with other treatment strategies. External validation showed satisfactory calibration and moderate discrimination (C-index: 0.61, 95% confidence interval 0.56-0.67). Patients who benefited most included women, those with stage IV, high body mass index and/or adenocarcinoma. The applicability of the model for guiding clinical decision-making was evaluated using decision curve analysis.Ī wide distribution of predicted gain in median PFS by pemetrexed-carboplatin over pemetrexed was found, with a median of 0.7 months (interquartile range: -0.1 to 1.5 months). The model was externally validated in the GOIRC 02-2006 trial. Using data of relapsed patients with advanced non-squamous NSCLC from the NVALT-7 trial, a Weibull model for prediction of gain in median progression-free survival (PFS) by pemetrexed-carboplatin was derived based on patient and tumor characteristics.
#NVALT 2 PLUS#
In this study, we developed and validated a prediction model for estimating absolute treatment effect of pemetrexed plus carboplatin versus single-agent pemetrexed in the second-line treatment of non-squamous non-small-cell lung cancer (NSCLC). Data from RCTs can be used for individualized treatment effect prediction, to identify patients who benefit from specific treatments. Translating results from randomized clinical trials (RCTs) to individual patients in clinical practice is challenging, as treatment effects can vary substantially among individuals.


 0 kommentar(er)
0 kommentar(er)
